We work on a diverse range of projects, from understanding the biomechanics of vascular pathologies to biomedical device development.
Microfluidics: Arterial Thrombosis and Anti-Thrombotic Therapy

Background
- Arterial thrombosis leads to myocardial infarction and stroke, which are two of the leading causes of death in the United States
- Three factors contribute to this condition: 1) high shear rate in stenotic vessels, 2) pro-thrombogenic collagen surfaces, and 3) the blood biochemistry of the individual
Our Findings
- Microfluidic assay variability is highly dependent on the geometry of the flow channel, over other factors of collagen coverage and anti-coagulation
- Shear independent platelet accumulation can be modulated by nanoparticles
Current Objectives
- Determine the intrinsic factors of blood that impact assay variability
- Optimize the nanoparticle size, material, and dosing for anti-thrombotic efficacy
Publications
Surface and Shear on Thrombogenicity
Background
- Thrombosis in medical devices is poorly understood and difficult to predict
- Both surface and shear rate drive thrombus formation, although this process is not well known, leaving it difficult to predict device thrombogenicity
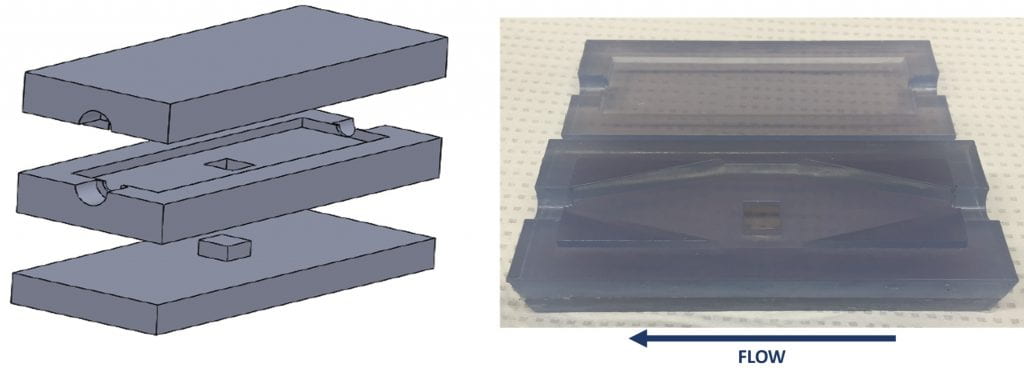
- To this end, we are designing parallel plate flow chambers in order to elucidate the relative contributions of material surface and shear rate to thrombosis
Proposed Methods
- The design of the chambers features a material insert and an interchangeable part to control for shear rate. The materials to be tested include acrylic, PVC, stainless steel, Dacron, and PTFE. The chamber will be perfused at shear rates of 50, 500, and 5000 1/s
- The chambers will be perfused via a recirculating system with porcine blood dosed to prevent infection and maintain cell survival. The temperature of the system will be maintained at 37 °C.
- Throughout circulation the blood will be assayed for coagulation, fibrinogen, platelet health and count, and hemolysis
Prosthetic Venous Valve
Background
- There are 1.4 million Americans suffer CVI (Chronic Venous Insufficiency) which can cause leg pain, varicose veins, fatigue, induration, and ulceration
- Existing treatments (compression stockings, vein stripping, and others) are not fully effective and do not restore normal venous functions
- A prosthetic venous valve could improve lives for 4 million patients
Our Findings
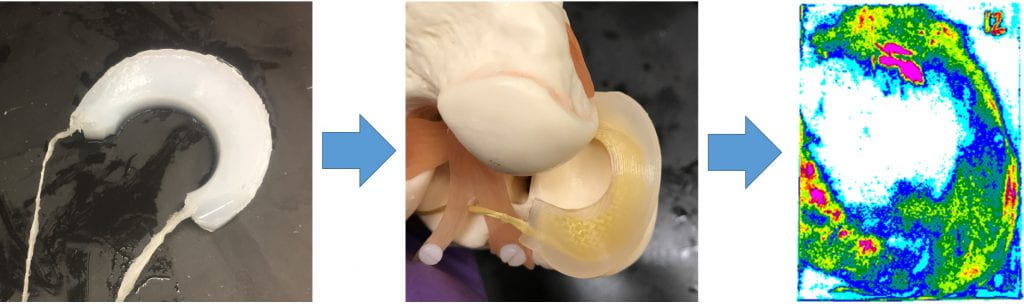
- Past designs attempted for in vivo animal test and failed because of thrombosis
- We developed a PVA (polyvinyl alcohol) prosthetic vein valve that is flexible enough to open & close properly with the clinical pressure difference
Current Objectives
- Develop a in vitro test methodology to evaluate a prosthetic venous valve
- Conduct in vivo animal studies and subsequently clinical trials prior to device launch
Prosthetic Meniscal Implant
Background
- Meniscus: load bearing and distribution, shock absorption, joint stabilizers
- Meniscal tears, lesions, or degeneration
- Causes: sports-induced, trauma, deterioration of tissue due to aging
- Symptoms: pain, functional impairment, joint tenderness, swelling
- 850,000 meniscus related surgeries in USA annually
- Current treatments:
- Removal of meniscus – knee joint instability and cartilage damage
- Meniscal allograft – limited supply and issues with size matching
- No commercial prosthetic meniscal implant exists today
Our Findings
- Previous synthetic menisci have insufficient mechanical, wear, or fixation properties
- Need a combination of material and structure/geometry to mimic natural meniscus
Current Objectives
- Design and evaluate a prosthetic meniscal implant
- Iteratively test materials and prototypes in an attempt to optimize properties
Medical Device Hemocompatibility

Background
- Thrombogenicity is the potential for blood-contacting medical devices to form blood clots
- Challenges of in vitro device simulation include long lengths of time required for meaningful results, the selection of clinically relevant endpoints, and recreation of an adequate analog on the benchtop
Our Findings
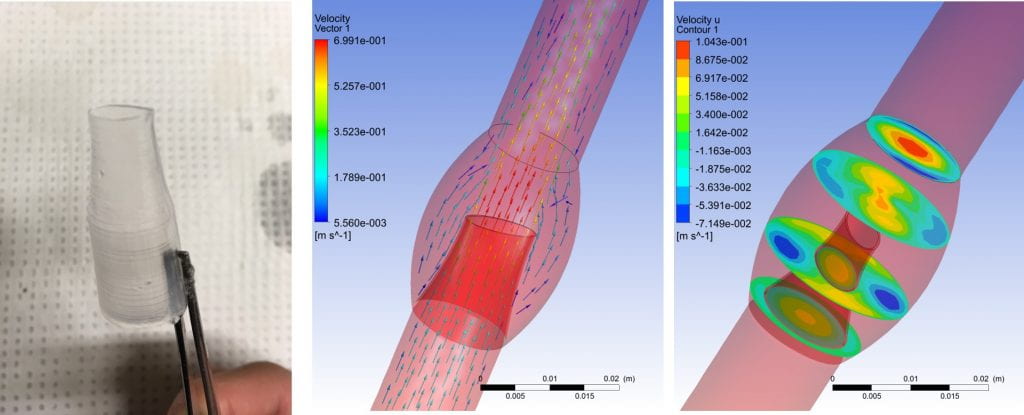
- The tubing connectors in ECMO circuits are a major source of thrombosis
- Common centrifugal pump has a physical stainless steel bearing that is exposed near the top of the pump that exhibits thrombi clinically
- Successfully recreated both thrombus in ECMO connectors and centrifugal pump with an in vitro benchtop setup
Current Objectives
- Test levitated centrifugal pumps without physical bearings
- Reduce thrombogenicity through novel connector design

